This white paper explores why Polypropylene mesh and pipe turn brittle after only a few years.
A WHITEPAPER
The following whitepaper is the property of Plastic Expert Group and cannot be copied or distributed without prior permission. Our analysis does not imply that specific plastic failures will happen systematically. Every case is unique and should be treated accordingly
PROBLEM:
Polypropylene (PP) PP is an Inherently Unstable Material
The 1998 edition of “Polypropylene; The Definitive User’s Guide and Databook”[1] teaches the following:
- “Polypropylene is highly susceptible to oxidation due to the presence of the tertiary hydrogen on the carbon atom bonded to the pendant methyl group. Polypropylene undergoes oxidation more readily than polyethylene, and oxidative chain scission, which reduces the molecular weight, under normal processing conditions.” Page 6
- “The decrease in molecular weight resulting from chain scission produces a gradual loss of mechanical properties.” Page 7
- “Stabilization of polypropylene is essential in order to prevent oxidative degradation. Because of the high susceptibility to oxidation due to the pendant methyl group, unstabilized polypropylene can begin to decompose almost immediately after formation. At elevated temperatures and in the presence of air, polypropylene disintegrates to an oxidized powder.” Page 27
Polypropylene (PP) is one of the highest volume and lowest cost plastics in the world. PP is inherently oxidatively unstable forcing manufacturers to add antioxidant stabilizers to their PP as soon as it is manufactured to keep the plastic from reacting with oxygen in the air causing the plastic to degrade and become brittle. Due to its low cost and its inherent poor longevity, PP is commonly used to manufacture single use/disposable items. In order for PP to be used to make products for long term use, high levels of antioxidant stabilizers must be added. Since antioxidants degrade as they inhibit oxidation, using PP to manufacture products for long term use, is challenging. PP products with high surface area that are used in applications where they are exposed to a strongly oxidizing environment, often degrade during use and fail to meet the intended service life.
[1] C. Maier and Teresa Calafut, “Polypropylene; The Definitive User’s Guide and Databook, Plastics Design Library, William Woishnis, Editor, NY (1998).
Why is Polypropylene Oxidatively Unstable?
PP is plastic composed of only carbon and hydrogen. PP belongs to a family of plastics called polyolefins. Polyolefins include polyethylene (PE), PP, ethylene/propylene (EP) copolymers, and copolymers containing ethylene, propylene and other olefins. Most polyolefins are semicrystalline. Crystallinity in polyolefins increase their strength, heat resistance, and solvent resistance properties. PE is inherently the most oxidatively stable of the polyolefins because PE is a polymer containing almost entirely -CH2– units. PP, on the other hand, contains – CHCH3- units. The tertiary H atoms (see the bolded H), on every other carbon atom along the polymer chains, are much more reactive than the H atoms in PE. Exposure of PP to oxygen, in the absence of antioxidant stabilizer, leads to oxidative degradation and embrittlement of the PP. As long as antioxidant stabilizers are present on the surface of PP parts, oxidation of the surface is inhibited. However, antioxidants eventually are depleted from the surface as they perform their function.[2] Once the antioxidants are depleted from the surface, oxidative embrittlement rapidly takes place.
[2] Duane Priddy, et al., Permanence of polymer stabilizers in hostile environments. Journal of Applied Polymer Science, 54(11), pp. 1605-12 (1994).
Effect of Copper on polypropylene Oxidation AND When it Became Known
It was established 60 years ago that exposure of PP to copper rapidly accelerates oxidative embrittlement.[3],[4],[5] Since copper and copper alloys are ubiquitous in potable water plumbing systems, it is not surprising that PP pipes used in potable warm water service, is prone to oxidation and embrittlement. The premature failure of PP piping used in warm water service was first reported in 1995 at an international plastic pipe conference in Edenborough.[6] A warm water piping system experienced 71 failures in the first four years of operation. Ten years later, the German insurance institute published an article entitled: “Wrong Operation or Systemic Deficiency? – Accumulation of Damage to Hot Water Pipes.”. They found that the PP pipe “prematurely aged which resulted in the material becoming brittle without any external chemical influences”. The article stated that there was no evidence that any of the failures were caused by excessive temperatures or pressures.[7] Although it was alleged that copper may have been involved in the premature failure of the PP pipe, it was never proven. At least one PP pipe manufacturer includes a “metal deactivator” additive in their formulation. However, the efficacy of the additive in reducing the catalytic effects of copper in PP pipe, used in warm water service, has not been confirmed or proven.
[3] C. Tholstrup, New inhibitors of copper-catalyzed oxidation of polypropylene, Proc. Battelle Symp. Thermal Stability Polymers, 1963, pp. 1 – 8 (1963).
[4] Ralph Hansen, The effects of morphology, antioxidants, and copper on the oxidation of polypropylene, Proc., Symp. Polypropylene Fibers, pp. 137 – 182 (1964).
[5] Ralph Hansen, et al., Inhibition of the copper-catalyzed oxidation of polypropylene, J. Polym. Sci, 2, pp. 587 – 609 (1964).
[6] D. Wagner, et al., Assessment of the reliability of plastic pipes in potable water installations affected by microbially influenced corrosion, Plastic Pipes Conference Association Papers Edenborough, pp. 497 – 505 (1995).
[7] Institute for Loss Prevention and Damage Research Public Insurance Companies June 2005 IFS newsletter.
Is Polypropylene Pipe Really “Chemically Inert?
PP pipe manufactures advertise their pipe as being “chemically inert” and that the piping will last for over 50 years. Unfortunately, PP is not chemically inert. As discussed above, if exposed to warm chlorinated water containing traces of copper, it may fail after only a few years of service. Since copper is ubiquitous in potable water systems, it is not surprising that many PP piping system failures have been and continue to be reported.[8],[9]
[8] See article Lawsuit filed in King County Superior Court alleges Aquatherm has been selling faulty pipes
[9] See the Aquatherm Pipe Lawsuit Investigation
Why does Polypropylene Pipe Fail and yet Pass ASTM F3497
PP pipe is required to pass the ASTM F3497 chlorine resistance test. The test exposes the pipe samples to purified, chlorinated hot water and measures the time to embrittlement of the pipe. The lab test time is accelerated by heat and pressure. The test results are then extrapolated to predict a service life. To pass the test, the predicted service life must be greater than 50 years. The problem with the test is that purified water is used. Since trace metals, including copper, are typically present in most municipal potable water supplies, and traces of copper are known to accelerate oxidative embrittlement of PP, it should not be surprising that some PP piping systems are prematurely failing after only a few years of service.
Choosing an inherently oxidatively unstable plastic to manufacture products that are required to last several decades, when used in services where the surface of the plastic is exposed to oxidizing agents, and then claim the products are “chemically inert”, is not prudent. Over the past 10 years, Plastic Expert Group has performed forensic failure analysis investigations on PP pipes and PP mesh implants. Both PP mesh and PP pipe are advertised as being “chemically inert”. Both fail in a similar way. The surface of the plastic turns brittle after only a few years of service. The embrittled surfaces appear very similar as shown below.
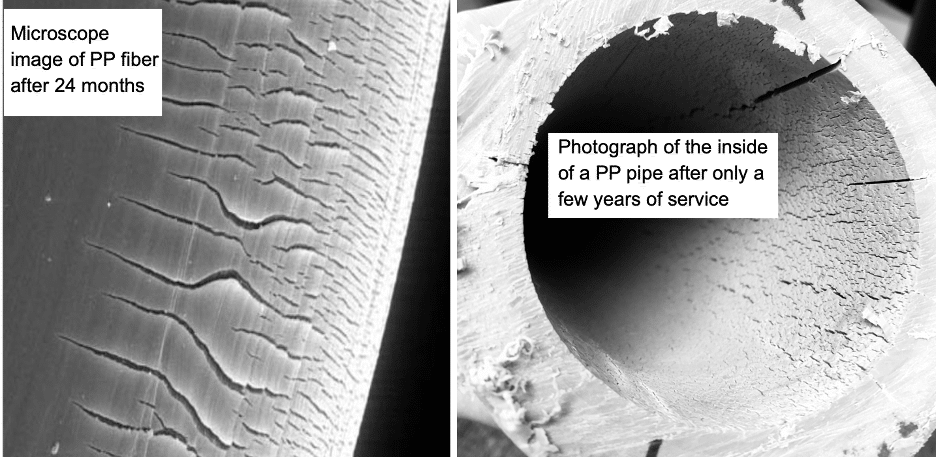
While fibers have higher surface area than pipe, the effects are the same. The mesh fiber surface and the inside pipe surface are both exposed to fluids containing oxidants which degrade and extract antioxidants leaving the surface unprotected and unstable. Mesh fiber implant surfaces are exposed to oxygenated blood and peroxidase enzymes, while in pipes, the inside surface is exposed to chlorinated disinfectants and trace metals including copper. During a 60 Minutes episode on Gynecological mesh implants in 2018, Dr. Priddy was interviewed by Scott Pelley. Mr. Pelley asked about the properties of PP. Dr. Priddy explained that PP is an oxidatively unstable plastic stating: “it is beyond my wildest imagination that anyone would think it is a good idea to implant PP mesh for long term use in the human body.” A year after that interview (2019), the FDA banned the sale of Gynecological mesh manufactured using PP.[10]
On June 29, 2023 a jury, in the King County v Aquatherm PP pipe case, found in favor of King County and awarded King County $18 million. Aquatherm PP piping has been installed in many buildings across the US and this situation may have national implications.[8]
We at Plastic Expert Group are highly experienced in determining the cause of failure of all plastic parts. When it comes to plastic plumbing, we are especially experienced having analyzed and tested thousands of plastic pipes including ABS, CPVC, HDPE, PEX, PP-R, and PVC pipes.